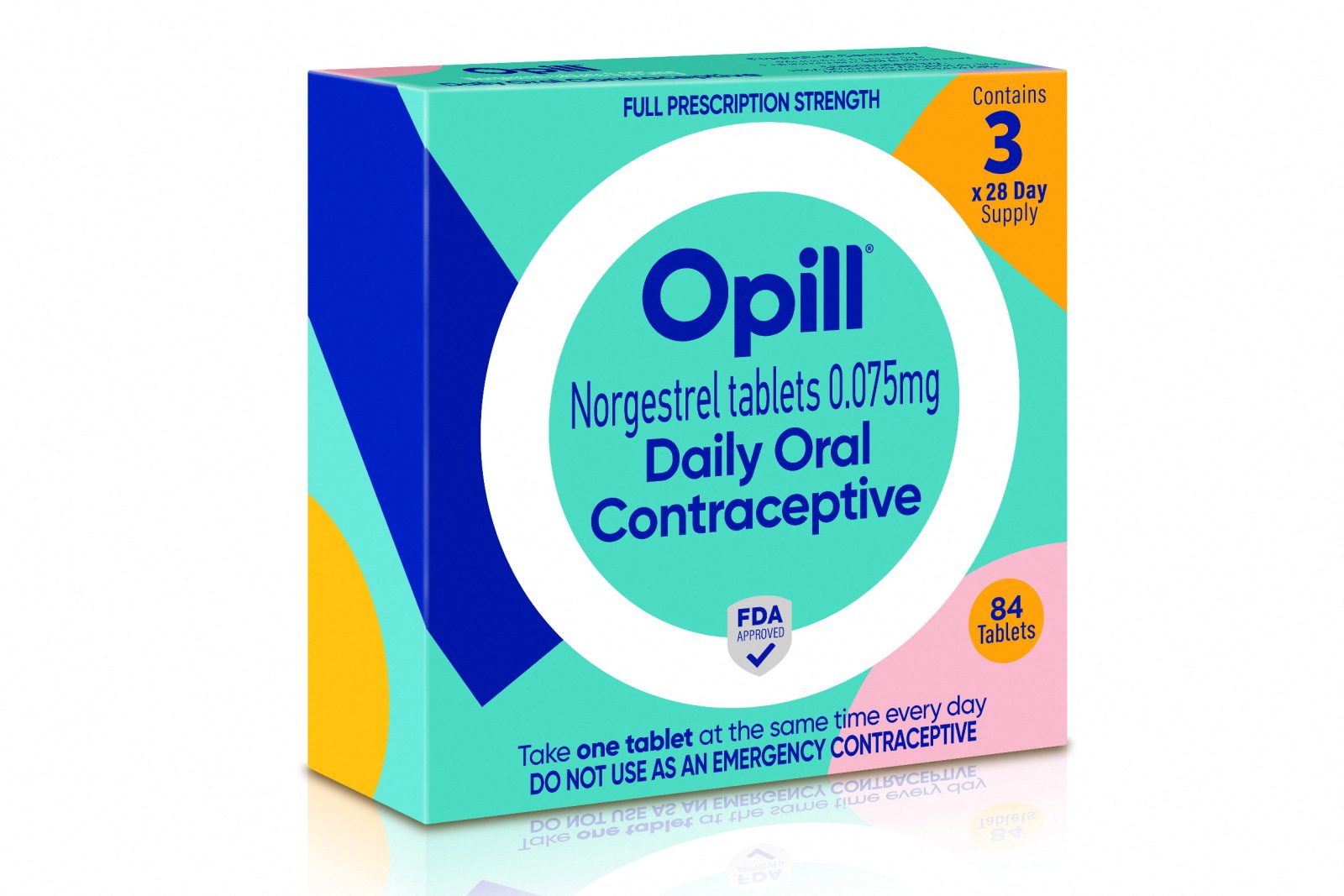
In a groundbreaking move, the Food and Drug Administration (FDA) has granted approval for the sale of a birth control pill without a prescription in the United States. This historic decision paves the way for enhanced accessibility to contraception, particularly benefiting young women, teenagers, and individuals who face challenges in obtaining a doctor’s prescription. The newly approved pill, named Opill, offers superior efficacy compared to other nonprescription birth control methods like condoms and spermicides. With an expected availability in early 2024, Opill’s manufacturer, Perrigo Company, aims to make the medication accessible, affordable, and inclusive for women of all ages.
Body: The FDA’s approval of Opill marks a significant milestone as it becomes the first nonprescription daily oral contraceptive available to millions of people in the United States. According to Dr. Patrizia Cavazzoni, the director of the FDA’s Center for Drug Evaluation and Research, when used as directed, Opill is a safe and highly effective method of preventing unintended pregnancies, surpassing currently available over-the-counter contraceptive options.
The decision to make a nonprescription birth control pill available to all age groups has received widespread support from reproductive health specialists, adolescent health experts, and esteemed medical organizations such as the American Medical Association, the American College of Obstetricians and Gynecologists, and the American Academy of Family Physicians. A survey conducted by the health care research organization KFF revealed that over three-quarters of reproductive-age women favored an over-the-counter pill due to its convenience, and nearly 40% expressed their willingness to use it. The survey also highlighted that women already taking birth control pills, those without health insurance, and Hispanic women were more likely to opt for this product.
In a surprising turn, many anti-abortion groups have refrained from criticizing the availability of over-the-counter birth control. While opposition primarily comes from certain Catholic organizations and Students for Life Action, the move to make contraception more accessible has gained widespread acceptance.
Opill, which has a long history of safety and efficacy and was approved for prescription use five decades ago, is identical to the prescription version. It boasts an effectiveness rate of 93% in preventing pregnancy with typical use. The FDA’s panel of scientific advisers, consisting of renowned experts in various relevant fields, unanimously voted in favor of making the birth control pill available without a prescription due to its substantial benefits outweighing the associated risks. Experts emphasized the urgent public health need for an over-the-counter option, considering the high prevalence of unintended pregnancies in the country.
Affordability remains a key concern for proponents of over-the-counter birth control pills. While the Affordable Care Act mandates health insurance plans to cover prescription contraception, over-the-counter methods are not included. Although some states have laws requiring coverage for over-the-counter birth control, the majority do not. The KFF survey found that 10% of women would be unable or unwilling to pay any out-of-pocket costs, while approximately 40% would pay $10 or less per month, and a third would pay $20 or less. To ensure equitable access, it is crucial to make over-the-counter birth control pills affordable and covered by insurance.
Opill is classified as a “mini pill” since it contains only one hormone, progestin, unlike combination pills that contain both progestin and estrogen. Cadence Health, a manufacturer of combination pills, is also exploring the possibility of applying for over-the-counter status with the FDA.
During the evaluation process, the FDA raised concerns about women with medical conditions, such as breast cancer and undiagnosed vaginal bleeding, potentially disregarding the warnings and using the product. Additionally, questions were raised regarding the ability of younger adolescents and individuals with limited literacy to follow the pill’s instructions. However, the FDA concluded that the individual risk for consumers is minimal, especially if they read and adhere to the labeling instructions.
Perrigo reported encouraging results from a study, with participants taking Opill correctly on 92.5% of the designated days. Participants who missed a pill reported following label instructions, such as abstaining from sex or using a condom. Among 955 participants, only six became pregnant while using Opill. These findings suggest that making the pill available over the counter could alleviate barriers to adherence, particularly for those who struggle to access timely resupply sites.
Conclusion: The FDA’s approval of the first over-the-counter birth control pill in the United States marks a major advancement in reproductive healthcare. Opill’s availability without a prescription will significantly improve accessibility, particularly benefiting young women, teenagers, and those facing barriers in obtaining a doctor’s prescription. By ensuring affordability and equitable coverage, over-the-counter birth control pills have the potential to be a game-changer, addressing systemic health inequities and reducing unintended pregnancies. This milestone decision contributes to a more inclusive and comprehensive approach to reproductive health.